
Carbonation of concrete
Carbonation of concrete is the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in the concrete to give mainly carbonates.
The formation of carbonates in these chemical reactions leads to a permanent sequestration of this CO2. Carbonation occurs from the CO2 exposed surfaces of the concrete structures into the concrete as shown in the figure below.
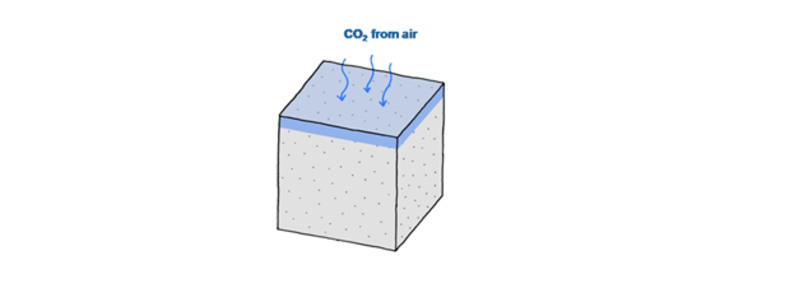
Carbonation from one CO2 exposed surface of a concrete structure.
This process slowly changes the pH in the concrete, starting at the outer layers, from about 13 to between 8-9. In reinforced concrete construction, this change in pH can disrupt the corrosion passivation of the steel reinforcement and, if moisture and oxygen are available, the steel reinforcement can start to corrode. This can lead to spalling of the concrete surface and a loss of mechanical strength. To avoid corrosion of the steel reinforcement, design rules prescribe appropriate concrete cover, typically over 30 mm, using a high strength concrete (therefore less porous). An example of such a situation is shown in the figure below.
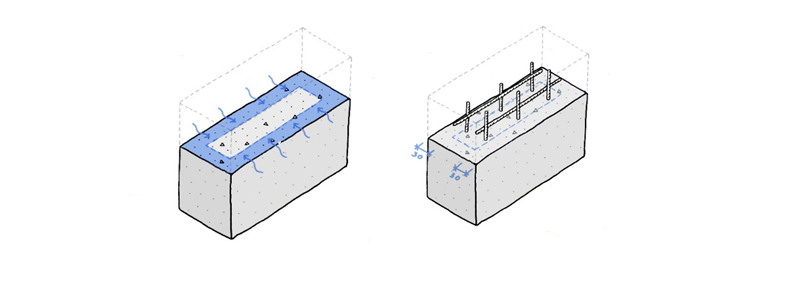
Carbonation reduces the pH-value of concrete. A high pH is needed to protect the reinforcement against corrosion. This is solved by designing using high strength concrete with a cover layer, typically 30 mm or more.
However, carbonation can also increase both the compressive and tensile strength of concrete, not considering the steel reinforcement. This effect can e.g. be used to improve the strength of crushed concrete when used as recycled aggregate in roads and foundations by allowing the material to carbonate. This carbonation process can be accelerated by storing crushed concrete and using it in such a way that the concrete surfaces are maximally exposed to CO2/air during the entire storage and use time. Mixed coarse and fine fractions in crushed concrete lead to particle packing and prevent air circulation as does dense covering layers. The handling and storage of crushed concrete for CO2 uptake is an important factor to maximize CO2 sequestration. An example of this is shown in the figures below.
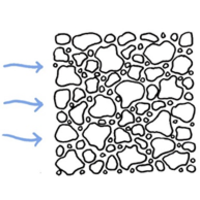
A wide range of crushed concrete particle sizes results in a compact packing structure with limited air movement.
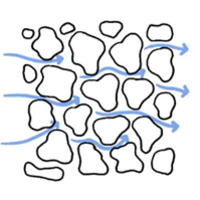
A reduced range of crushed concrete sizes allows packs less efficiently and thus allows air and CO2 to move through the structure more readily, increasing carbonation rate. Alternatively, periodic mixing of the particles may also expose the surfaces of the particles to CO2 to facilitate carbonation.
Climate Aspects of Carbonation of Concrete
The climate issue is important both in an international perspective and nationally. Many organizations and companies are actively working on climate issues and greenhouse gas reductions are often an important goal, as well as mapping and monitoring of greenhouse gases.
At the international level, different countries report greenhouse gas emissions to the United Nations Framework Convention on Climate Change (UNFCCC). The international monitoring of net greenhouse gas emissions to the atmosphere is an important part of the mapping and monitoring of global climate efforts. The guidelines for how emission calculations are to be carried out are presented in the framework of the Intergovernmental Panel on Climate Change (IPCC) and are regulated in, for example, the document 2006 IPCC Guidelines for National Greenhouse Gas Inventories.
In order for the calculations of net greenhouse gas emissions to the atmosphere to be as accurate as possible, it is important that the guidelines and other documents be updated and improved by the IPCC. Consequently, it is important to improve methodology and to update the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. This Guideline is, for the moment, not updated to include carbonation of concrete. Thus, other sources have to be used when such calculations are to be performed. Such calculations are also relatively complex and require a significant technical and scientific basis to be able to perform correctly. In addition, computer models may also be needed to enhance the calculations and to improve the accuracy. An important purpose of this website is to provide such supporting material and such computer-aided calculation models.
Carbonation – How does it work?
The most common cement type, Portland cement, is made by mixing limestone with other materials such as iron, aluminum and silicon containing minerals, often in the form of clay. Other materials may also be present in cement manufacture such as blast furnace slag and fly ash. These materials are examples of the use of recycled materials in the manufacture. The materials are ground, mixed and sintered at high temperature (1400-1450ºC) in a rotary kiln to form cement clinker.
The production of clinker requires high temperature and is therefore energy intensive. The fuels used globally are mainly coal, oil and pet coke (from oil refining), but also fuels made from biomass and residues such as waste oil, solvents, plastic and scrap tires are increasingly used. The fossil fuels used in the cement kiln give rise to CO2 emissions (GHG). Emissions of other greenhouse gases, such as methane (CH4) and nitrous oxide oxide (N2O), are present only in small amounts since the combustion temperature is high and the combustion condition is well-controlled in the cement kilns.
In the production of cement, the vast majority of the carbon dioxide is formed, partly from the combustion of the fuels needed in clinker production (cement kiln) and partly from the calcination of the limestone in the raw material to the cement klin, e.g. according to the reaction:
CaCO3 → CaO + CO2
Reversible calcination - Carbonation
These calcination reactions are not permanent but reversible. This means that CO2 is absorbed into the concrete by a process referred to as carbonation. In principle, the same amount of CO2 driven off from the raw materials in the cement kiln can be taken up in the concrete by carbonation. However, the amount of CO2 that will be taken up by carbonation in a reasonable timeframe depends on several factors. The carbonation process is a slow process that can last for many years. The time aspect is thus an important issue. The availability of CO2 to the concrete is also crucial. The concrete must be exposed to CO2 to be able to carbonate. The transport of CO2 molecules into the concrete is thus also an important factor. For example, if the concrete is crushed after use, the carbonation rate will increase considerably due to the increased surface area to volume ratio.
Important aspect to take into account
Carbonation is thus an important aspect to take into account in climate and emission calculations for cement and concrete. Today, emissions of greenhouse gases from different countries are reported, which in turn are used to support different climate strategies. Reporting takes place nationally to national authorities (e.g. National Inventory Report, NIR) and internationally to the UNFCCC. Guidelines for the emission calculation are developed and kept updated by the IPCC. The current version, 2006 IPCC Guidelines for National Greenhouse Gas Inventories, covers greenhouse gas emissions from cement and concrete processes. Both CO2 emissions from fossil fuel combustion and emissions from the raw materials (calcination) are included
However, no consideration is given to the carbonation of concrete (although it is noted as an area for future work). This may be considered a shortcoming in these calculations, which can lead to less accurate results. An estimate is that the use of concrete today accounts for about 5-8 percent of the world's carbon dioxide emissions. About 50-60% of these emissions emanate from the raw materials and thus has a potential to be reabsorbed by carbonation of the concrete, partly during the use phase of the concrete products, and partly in the end-of-life and secondary use stage. We are convinced that this is an important part of climate calculations and we therefore want to improve the calculations to better reflect the reality. This web page proposes methods and models for calculating CO2 uptake (carbonation) in various cement-containing products.

